Securpharm – Players in the Security Network
You would like to know more?
Contact us
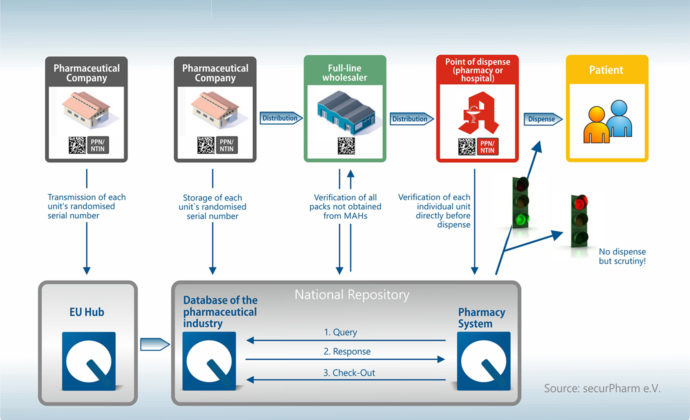
Who actually does what in the system?
The new EU Falsified Medicines Directive concerns all participants in the medicine supply chain in Germany and Europe – from the manufacturer to the wholesaler to the pharmacy. Each of these players takes on an important role in this security network with the goal of preventing the introduction of falsified medicines into the legal supply chain. To this end, medicine packagings are equipped with different security systems:
- Unique serial number – Each number is only assigned once
- Tamper-proof protection – Tab or seal

So, who actually takes on what role?
Each of the players makes an important contribution to the check. It all begins with the pharmaceutical manufacturer and ends with the handover of the medicine to the patient – usually in public pharmacies. As such, the pharmacy takes on a special role in the security network.
The manufacturer
The supply chain begins with the manufacturer, who generates a unique serial number in the production process and uploads it into a database (the European HUB – European Medicines Verification System). This serial number, together with the batch number and expiry date, are contained in the data matrix code, which is printed on the packaging.

The wholesaler
The wholesaler is only required to check the security precautions on packagings returned by pharmacies and other wholesalers as well as pharmaceuticals not supplied by the manufacturer or a wholesaler commissioned by the manufacturer. However, the batch tracking of documentation will become obligatory as of February 9. In other words, documentation requirements are increasing here too.
Reimporters and parallel dealers are required to check and book out medicines requiring verification during the export process.

The pharmacy
The pharmacy performs the security check in the final instance. This involves scanning of a bar code when handing the medicine over to the customer. The information is transmitted to the central safety HUB in real time and the number verified. If the result is positive, the packaging is approved and booked out of the system. In the case of a negative response, the packaging is retained by the pharmacy and returned to the wholesaler. Working with a pharmacy robot offers the advantage that the packagings are already checked during the incoming goods process, which helps avoid awkward situations at the sales counter.
Source: securPharm e.V.
You might also be interested in
Blackout in the pharmacy: What to do when nothing works anymore?
First Covid-19, then war in Europe, and now, an energy crisis. For years we have been moving from one crisis to the next, which was previously unthinkable. But, even if the first two are still red-hot and far from forgotten, the next topic is already waiting to be considered: ENERGY.
E-prescriptions and dispensing terminals
New opportunities for local pharmacies. E-prescription is bringing innovations that will simplify everyday pharmacy work.
Pharmacies’ last-mile delivery service – what will the e-prescription change?
Patients have focused more on Pharmacy last mile services during the global COVID-19 pandemic – how should pharmacies position themselves in the future?


