
FMD in Pharmaceutical Wholesale
You would like to know more?
Contact us
Interview with Guido Lübeck
What requirements will the FMD regulations entering into force on February 9, 2019, introduce for wholesale in particular? Guido Lübeck, Project Manager Alliance Healthcare Deutschland GmbH, Subsidiary GEHE Weiterstadt, reported on how GEHE is dealing with the topic.
Mr. Lübeck, Directive (EU) 2016/161 stipulates that pharmaceutical wholesale is obliged to check medicines requiring verification in order to ensure that they are genuine. What does that mean for you as a wholesaler precisely?
Guido Lübeck: The directive stipulates that we must verify all packagings returned by pharmacies and other wholesalers as well as pharmaceuticals not supplied by the manufacturer or a wholesaler commissioned by the manufacturer. In addition, we are also required to check all medicines to ensure they are genuine and subsequently deactivate them if they cannot be returned for sale and/or need to be destroyed. Furthermore, we must also take care of the ‘booking out’ of the product from the database for certain customer groups such as authorities, as these customers do not normally have access to the German securPharm system. Even though these are predominantly Rx products at this time, this translates to extra organizational efforts on our part, which we need to deal with using the available resources.
How have you prepared for this matter at GEHE in order to remain efficient and not experience any loss in productivity?
Guido Lübeck: The issue of pharmaceutical security is a top priority at GEHE. In the scope of the GDP guidelines, which came into force in 2013, we have already been confronted with higher documentation requirements as part of quality assurance efforts. As a result of this experience and in preparation for the FMD regulation, we began changing over our processes a few years ago already. Among other things, we have placed greater emphasis on higher automation quotas with regard to the stockroom and order picking. Our medium- and slow-moving items as well as some refrigerated medications are now managed via order picking systems. That makes our work considerably easier.
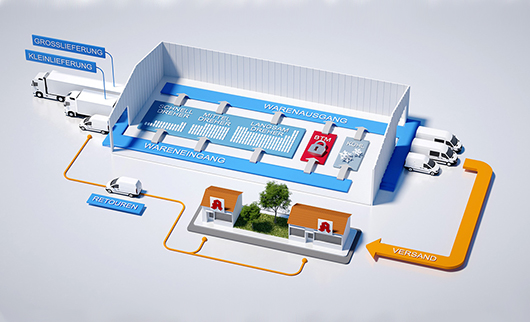
And how exactly does it make your work easier?
Guido Lübeck: In the past, batch handover was previously only legally required for special medications and types of customer. Since February 9, it has been compulsory for almost all Rx items. With the order picking system from BD Rowa, the effort associated with documenting the batch number and serial number is next to nothing. The system records the information contained in the 2D data matrix code via the scanner when adding the items to the stock and communicates this information directly to the ERP system. When the items are removed from the stock, the information is assigned to the orders in the ERP system via a real-time interface. This allows us to ensure seamless documentation at all times and track the package beyond the current legal requirements and even down to the serial number if necessary.
Has the introduction of the order picking system changed the workflow in your stockroom?
Guido Lübeck: We now intentionally store more Rx items in the system in order to keep the manual efforts required as low as possible. This is particularly evident to us in our other storage areas, for example fast-moving items, where batch tracing takes up considerably more time. We shall continue to monitor this and, if necessary, decide how we can further optimize the design of our stockroom.
You might also be interested in
More time for the essentials
Manual warehouse maintenance takes up a lot of valuable working time. Thanks to the immense time savings, a dispensing robot no longer only pays off for larger pharmacies.
Goodbye repeat deliveries - welcome delivery capability
Delivery capability is an important issue for pharmacies. By increasing this, a pharmacy robot saves valuable resources every day.
Finding and retaining good staff
A positive team spirit is the key to success in any pharmacy. Find out how you can improve this spirit.


